Artificial Intelligence in Health Care: Benefits and Challenges of Machine Learning Technologies for Medical Diagnostics
What GAO Found
Several machine learning (ML) technologies are available in the U.S. to assist with the diagnostic process. The resulting benefits include earlier detection of diseases; more consistent analysis of medical data; and increased access to care, particularly for underserved populations. GAO identified a variety of ML-based technologies for five selected diseases — certain cancers, diabetic retinopathy, Alzheimer’s disease, heart disease, and COVID-19 —with most technologies relying on data from imaging such as x-rays or magnetic resonance imaging (MRI). However, these ML technologies have generally not been widely adopted.
Academic, government, and private sector researchers are working to expand the capabilities of ML-based medical diagnostic technologies. In addition, GAO identified three broader emerging approaches—autonomous, adaptive, and consumer-oriented ML-diagnostics—that can be applied to diagnose a variety of diseases. These advances could enhance medical professionals’ capabilities and improve patient treatments but also have certain limitations. For example, adaptive technologies may improve accuracy by incorporating additional data to update themselves, but automatic incorporation of low-quality data may lead to inconsistent or poorer algorithmic performance.
Spectrum of adaptive algorithms
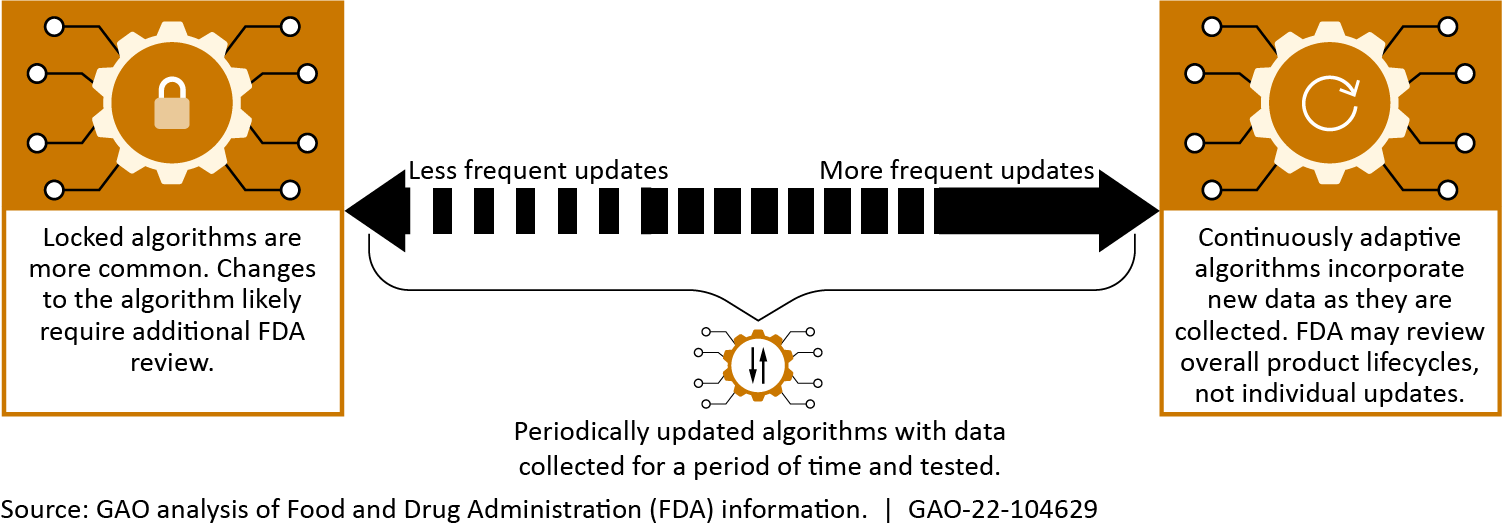
We identified several challenges affecting the development and adoption of ML in medical diagnostics:
- Demonstrating real-world performance across diverse clinical settings and in rigorous studies.
- Meeting clinical needs, such as developing technologies that integrate into clinical workflows.
- Addressing regulatory gaps, such as providing clear guidance for the development of adaptive algorithms.
These challenges affect various stakeholders including technology developers, medical providers, and patients, and may slow the development and adoption of these technologies.
GAO developed three policy options that could help address these challenges or enhance the benefits of ML diagnostic technologies. These policy options identify possible actions by policymakers, which include Congress, federal agencies, state and local governments, academic and research institutions, and industry. See below for a summary of the policy options and relevant opportunities and considerations.
Policy Options to Help Address Challenges or Enhance Benefits of ML Diagnostic Technologies
| Opportunities | Considerations | |
|
Evaluation (report Policymakers could create incentives, guidance, or policies to encourage or require the evaluation of ML diagnostic technologies across a range of deployment conditions and demographics representative of the intended use. This policy option could help address the challenge of demonstrating real world performance. |
|
|
|
Data Access (report Policymakers could develop or expand access to high-quality medical data to develop and test ML medical diagnostic technologies. Examples include standards for collecting and sharing data, creating data commons, or using incentives to encourage data sharing. This policy option could help address the challenge of demonstrating real world performance. |
|
|
|
Collaboration (report Policymakers could promote collaboration among developers, providers, and regulators in the development and adoption of ML diagnostic technologies. For example, policymakers could convene multidisciplinary experts together in the design and development of these technologies through workshops and conferences. This policy option could help address the challenges of meeting medical needs and addressing regulatory gaps. |
|
|
Source: GAO. | GAO-22-104629
Why GAO Did This Study
Diagnostic errors affect more than 12 million Americans each year, with aggregate costs likely in excess of $100 billion, according to a report by the Society to Improve Diagnosis in Medicine. ML, a subfield of artificial intelligence, has emerged as a powerful tool for solving complex problems in diverse domains, including medical diagnostics. However, challenges to the development and use of machine learning technologies in medical diagnostics raise technological, economic, and regulatory questions.
GAO was asked to conduct a technology assessment on the current and emerging uses of machine learning in medical diagnostics, as well as the challenges and policy implications of these technologies. This report discusses (1) currently available ML medical diagnostic technologies for five selected diseases, (2) emerging ML medical diagnostic technologies, (3) challenges affecting the development and adoption of ML technologies for medical diagnosis, and (4) policy options to help address these challenges.
GAO assessed available and emerging ML technologies; interviewed stakeholders from government, industry, and academia; convened a meeting of experts in collaboration with the National Academy of Medicine; and reviewed reports and scientific literature. GAO is identifying policy options in this report.
For more information, contact Karen L. Howard at (202) 512-6888 or [email protected].
