How Phiex could revolutionize medical device sterilization

CL Tian is co-founder and CEO of Phiex. [Photo courtesy of Phiex]
MedTech Innovator winner Phiex is functioning with professional medical device brands on an alternative to ethylene oxide (EtO) sterilization.
The new approach employs dry chlorine dioxide gasoline generated inside the health care device’s solution packaging. The powder turns into a microbe-destroying fuel when exposed to mild, Phiex co-founder and CEO CL Tian stated in an job interview.
Health-related gadget producers can either integrate the powder into their product packaging or as a secondary pouch. And so much, most equipment that can be sterilized with EtO can be sterilized with the Phiex method, Tian explained.
EtO is the leading sterilization strategy for professional medical units. Makers and contract sterilization corporations use EtO on additional than 20 billion healthcare devices every year, or approximately 50 percent of all units that need sterilization.
But the medtech marketplace and Food and drug administration are discovering possibilities to EtO thanks to protection issues about sterilization facility emissions. And business affiliation AdvaMed has warned that EtO sterilization potential is currently maxed out.
Chlorine dioxide as an alternate sterilization method
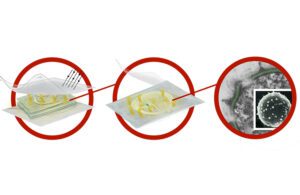
Phiex’s health care machine packaging sterilizes healthcare devices by releasing a dry chlorine dioxide gas when uncovered to ambient, wide-spectrum mild. The gas seeks the sure h2o in the mobile wall of germs and effectively oxidizes the cell membrane. [Illustration courtesy of Phiex]
Chlorine dioxide gasoline has been EPA-registered as a sterilant since 1988. It is by now used to sterilize health care equipment in airtight chambers and is also utilised for food and drinking water sterilization. The gasoline has not been linked to cancer or start problems, while the Fda has had to warn customers not to drink liquid chlorine dioxide products and solutions dangerously promoted as health-related treatments.
Tian characterizes Phiex’s in-packaging sterilization as a gentle system, with no need for humidity, temperature or strain pre-conditioning. Producing chlorine dioxide gas from a good-condition powder allows Phiex to manage the concentration of gasoline desired to sterilize a certain device inside of its packaging.
“We have a proprietary, dry condition powder that we combine instantly into the packaging,” she explained. “When it is uncovered to ambient gentle, there’s a response and we crank out what we contact a micro-environment of chlorine dioxide gas. … It has exceptional material compatibility, and that is been a important worry for the OEMs with any alternate sterilization modality.”
The Phiex method can also be used as an substitute to radiation or warmth sterilization methods, which can discolor or problems professional medical unit resources this sort of as silicone, polyurethanes, polyethylene, polyolefins and other delicate polymers.
“We haven’t observed a substance effect in our studies with our buyers. There is no transform in mechanical properties, molecular body weight, tensile strengths or coloration,” Tian said. “And that is even with us exposing it to considerably better concentrations of our dry — dry being the operative phrase — chlorine dioxide than we would anticipate in an precise business location.”
An additional advantage in excess of radiation sterilization is that Phiex’s sterilization course of action doesn’t harm electronics. That is useful for gadget builders who are integrating batteries and electronics for the first time into a machine that could beforehand be sterilized with gamma rays, for illustration.
Radiation could continue being the process of decision for brands that require to sterilize inside airtight compartments where EtO, chlorine dioxide and other gases can not reach. And although radiation and EtO sterilization want sterilization chambers and substantial amenities with pricey gear like particle accelerators, people approaches may possibly still make perception for bulk sterilization of pallets of hardy products.
“Where we enjoy effectively is at the device amount, sterilizing one particular or a pair inline,” Tian claimed. … “It’s a absolutely diverse way of considering about how we sterilize.”
How Phiex operates with makers
Phiex is currently creating customized-made packaging product to makers for their manufacturing strains, but Tian declined to identify clients.
“The business circumstance for going to a modality like ours — inline packaging-primarily based sterilization modality — is so appealing from a strategic advantage perspective that even if we wanted to, our prospects have been really purposely hush-hush about operating with us,” she stated.
Tian explained how Phiex will work with buyers using hypothetical Class II, Course II and Fda-cleared 510(k) units.
“You assemble it in your producing line, put it alongside one another and place it into your primary packaging, which commonly has a layer that is gasoline permeable if it’s currently sterilized with ethylene oxide,” Tian reported. “Let’s just say that shopper wants to use a secondary packaging, indicating right after they position it in their principal and seal it, they can area some range into the secondary pouch, seal that, and commence to sterilize it. Immediately after the cycle time is up, they can open it, put it into their paperboard carton, into their bins, and then assuming they launch the batch according to their launch protocol, that box is now completely ready to go off to their position of distribution or their end purchaser.”
That process, Tian explained, is weeks a lot quicker than sterilizing by 3rd-occasion vendors, where by it may possibly acquire times or weeks just to make adequate solutions to efficiently ship to a contract sterilizing facility. Relying on how backed up the deal sterilizer is, there could be more delays until the merchandise are sterilized and returned to the product producing plant or sent to a distribution heart.
“Most OEM individuals communicate about the total of time wasted with problems and problems that take place with sterilization, both from producing industrial products and solutions and also from getting novel products from the bench through the R&D method,” Tian reported. “There’s a whole lot of fascination in possessing a a lot more sustainable and far better-for-business enterprise sterilization modality.”
What is following for Phiex?
Phiex shoppers are in several phases of adoption, ranging from distributing up to date filings with the Fda to employing the technology into their producing lines. The 1st solutions sterilized with Phiex packaging could attain stop-buyers in late 2023 or early 2024.
Factors are shifting quick for the Boston-centered startup. Phiex had what Tian would only say was a “very constructive conversation with the FDA” last 12 months. Then the business won the $350,000 grand prize from the 2022 MedTech Innovator plan, which Tian explained was “hands down the ideal factor we have ever accomplished.”
“There’s a great deal of plans out there,” she reported of the global medtech accelerator. “What makes this distinctive is the persons in the plan. They’re some of the top rated leaders in medtech, they’re so supplying and willing to do the job with every single of the portfolio firms — not just us — making introductions, doing the job on the small business, sharing best tactics in a genuinely open, generous way. A single factor that we have in scarcity as startups is time, so any way to discover from what’s worked in advance of, study from successes instead than our individual encounter, is so a must have. And I have truly appreciated that group that [CEO Paul Grand] and the group have cultivated.”
What does the foreseeable future keep for Phiex?
“If you experienced questioned me exactly where I thought we would be this time previous yr, I couldn’t have imagined that we have gotten this much and this significantly unsolicited industry desire and engagement with the main OEMs and important contract makers,” she claimed. “I try out to keep out of the small business of predicting, due to the fact I have a suspicion that we’re heading to go at a a great deal more quickly clip than we’re conservatively scheduling.”
